Most Commented
Clinical Trials: Gcp, Ethics & Compliance Mastery




Description material

Clinical Trials: Gcp, Ethics & Compliance Mastery
Published 5/2024
MP4 | Video: h264, 1920x1080 | Audio: AAC, 44.1 KHz
Language: English
| Size: 3.86 GB[/center]
| Duration: 6h 3m
Navigate the complexities of clinical trials with expertise in GCP, ethical practices, and compliance.
What you'll learn
Understand the ethical considerations and regulatory framework governing clinical research.
Master the process of study design, protocol development, and informed consent in accordance with regulatory guidelines.
Gain proficiency in implementing Good Clinical Practice (GCP) guidelines and ensuring compliance throughout the research process.
Navigate regulatory requirements for conducting clinical trials, including FDA regulations and international standards.
Learn best practices for data management, record-keeping, and addressing challenges related to informed consent.
Develop skills in monitoring and reporting clinical trials effectively to maintain data integrity and participant safety.
Requirements
Basic understanding of clinical research terminology and processes is beneficial but not mandatory.
Description
Welcome to Clinical Trials: GCP, Ethics & Compliance Mastery, a course meticulously designed for professionals looking to deepen their understanding and ensure the highest standards in the world of clinical research. In this course, you will explore the ethical, regulatory, and practical aspects critical to the successful management of clinical trials.The course begins with an introduction to the fundamentals of clinical research, providing insights into the roles and responsibilities of various stakeholders, along with an overview of the ethical and regulatory frameworks that guide clinical studies. You will learn about the pivotal aspects of Good Clinical Practice (GCP), which is essential for ensuring patient safety and the integrity of clinical data.As we dive deeper, you will become adept at designing clinical trials, developing robust study protocols, and mastering the informed consent process. This includes understanding how to effectively communicate with diverse participant populations, addressing ethical challenges, and ensuring participants' rights and welfare are protected.The module on regulatory compliance covers vital topics such as FDA regulations, international standards, and how to prepare for audits and inspections. You'll also gain skills in data management, record-keeping, and understanding the intricacies of monitoring and reporting to maintain adherence to all necessary regulations.By the end of this course, you will be equipped to implement GCP guidelines confidently, manage clinical research projects efficiently, and navigate the complexities of ethical and regulatory compliance in clinical trials. Whether you're a budding clinical researcher or a seasoned professional, this course will enhance your capabilities and help you to contribute effectively to the field of clinical research.
Overview
Section 1: Foundations of Clinical Research and Regulatory Framework
Lecture 1 Ethical Considerations in Clinical Research
Lecture 2 Importance of Good Clinical Practice
Lecture 3 Understanding Regulatory Framework in Clinical Research
Lecture 4 Roles of Coordinators and Investigators
Lecture 5 Clinical Trial Success: Recruitment, Challenges, and Retention
Lecture 6 Quality Assurance in Clinical Research
Section 2: Study Design, Protocol Development, and Informed Consent
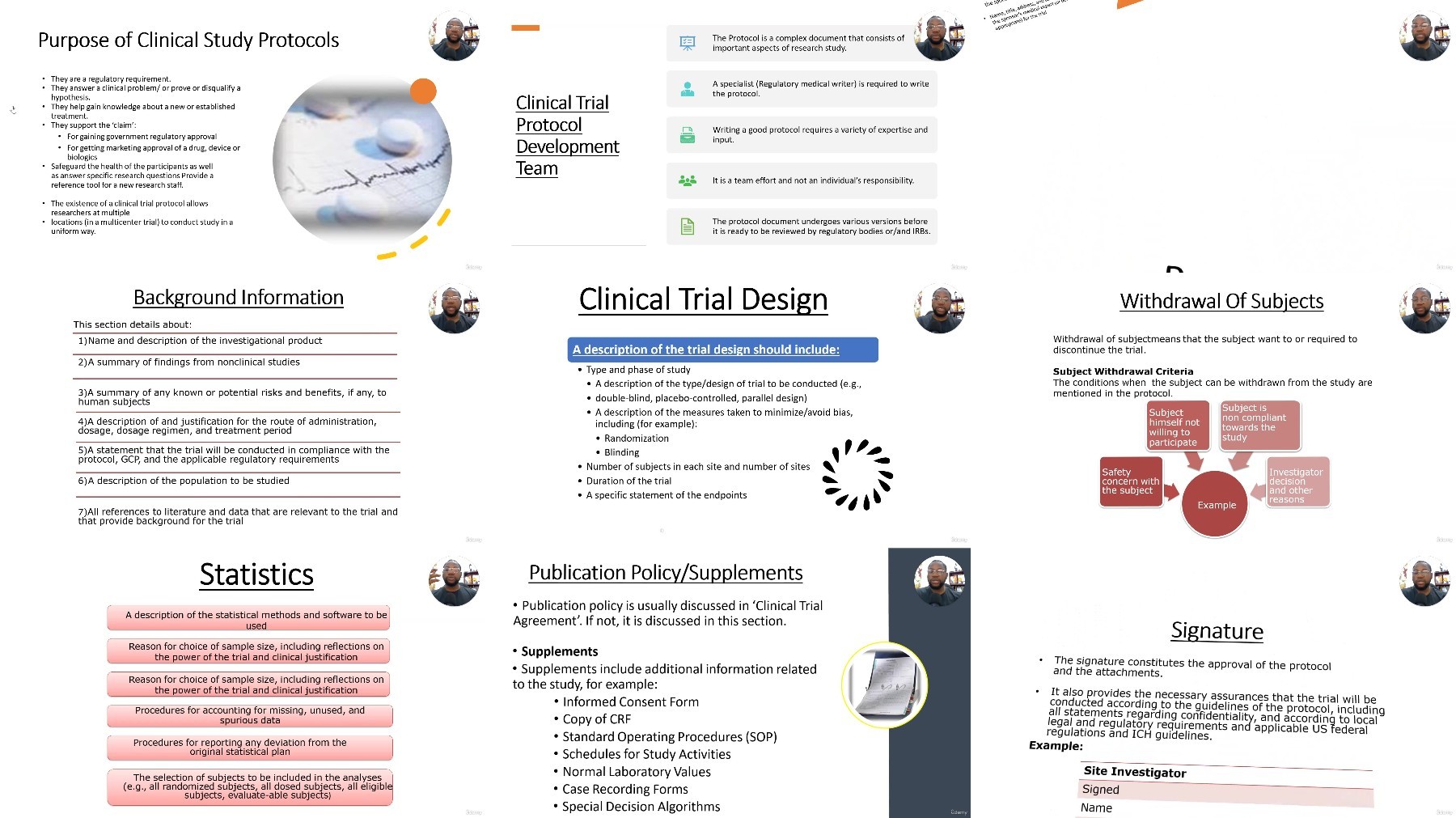
Lecture 7 Protocol Development and Key Elements
Lecture 8 Protocol Amendments and Deviations
Lecture 9 Overview of Clinical Research Study
Lecture 10 Understanding Clinical Research Protocols
Lecture 11 Ensuring Inclusivity and Diversity
Lecture 12 Essential Documents in Clinical Research
Section 3: Good Clinical Practice Guidelines and Compliance
Lecture 13 Good Clinical Practice Guidelines
Lecture 14 Institutional Review Boards and Ethics Committees
Lecture 15 FDA Regulations and Guidelines in Clinical Research Conduct
Lecture 16 ICH-GCP Principles
Lecture 17 GCP Compliance and Quality Assurance
Lecture 18 GCP Audit and Inspection: Understanding the Differences
Section 4: Regulatory Compliance in Clinical Research
Lecture 19 Regulatory Affairs in Clinical Research
Lecture 20 FDA Regulations for Clinical Trials
Lecture 21 International Regulatory Requirements
Lecture 22 Protocol Amendments and Clinical Trials
Lecture 23 Importance of Safety Reporting
Lecture 24 Safety Reporting and Adverse Events
Section 5: Data Management, and Record Keeping Challenges
Lecture 25 Clinical Data Management
Lecture 26 Data Collection in Clinical Research
Lecture 27 Essential Docs In Clinical Research
Lecture 28 Electronic Data Capture System in Clinical Research
Lecture 29 Data Validation and QC in Clinical Research
Lecture 30 Understanding the Essentials of TMF
Section 6: Monitoring and Reporting in Clinical Trials
Lecture 31 Introduction to Clinical Trial Monitoring
Lecture 32 Clinical Research Monitoring: Importance and Actions
Lecture 33 Understanding the Role of CRC and Investigators
Lecture 34 Strategies for Ensuring Compliance and Conducting MV
Lecture 35 Research Monitoring: Understanding Roles and Responsibilities
Lecture 36 Site Selection in Clinical Trials
Clinical research coordinators,Healthcare professionals involved in clinical trials,Individuals interested in pursuing a career in clinical research
https://fikper.com/97jqt7vbbu/Clinical.Trials.GCP.Ethics.Compliance.Mastery.z01.html
https://fikper.com/oYDG1Q9x7K/Clinical.Trials.GCP.Ethics.Compliance.Mastery.z02.html
https://fikper.com/H2fsKxwum6/Clinical.Trials.GCP.Ethics.Compliance.Mastery.zip.html
https://rapidgator.net/file/ededebf118ffb3bd5277cafb218af362/Clinical.Trials.GCP.Ethics.Compliance.Mastery.z01
https://rapidgator.net/file/de50052c7e2eda0cb024bbc2ec6dc6d1/Clinical.Trials.GCP.Ethics.Compliance.Mastery.z02
https://rapidgator.net/file/4c6de847a056a0f0f18f313fe433ea1d/Clinical.Trials.GCP.Ethics.Compliance.Mastery.zip
Free search engine download: Clinical Trials GCP Ethics Compliance Mastery
Join to our telegram Group
Information
Users of Guests are not allowed to comment this publication.
Users of Guests are not allowed to comment this publication.
Choose Site Language
Recommended news
Commented


![eM Client Pro 9.2.1735 Multilingual [Updated]](https://pikky.net/medium/wXgc.png)



![[PORTABLE] Softany WinCHM Pro 5.491](https://i.postimg.cc/xTMss02F/Win-CHM-Pro.png)


![Movavi Video Editor 24.0.2.0 Multilingual [ Updated]](https://pikky.net/medium/qhrc.png)

